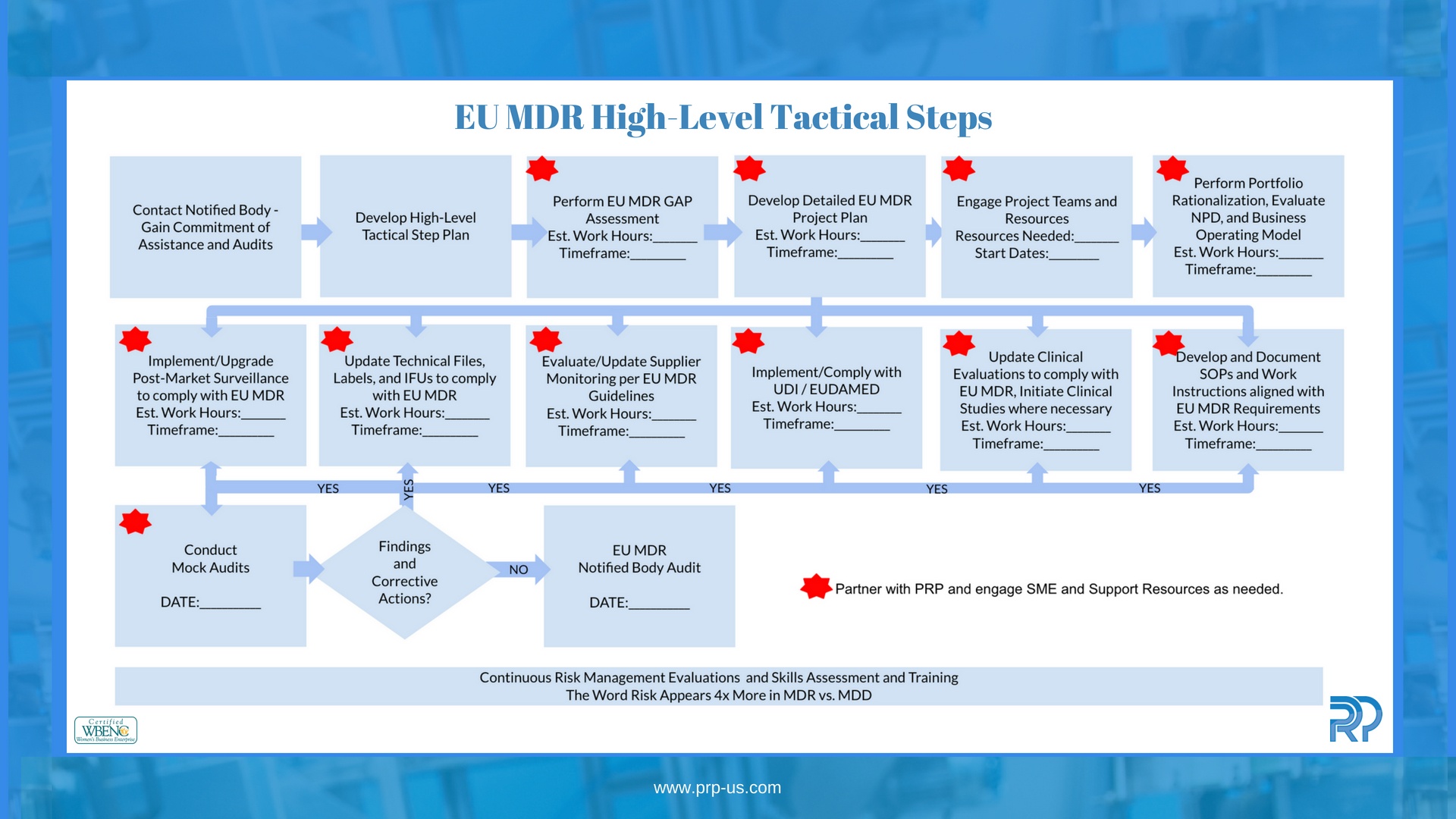
EU MDR High-Level Tactical Steps to Help You Start You on Your Journey!
The European Union Medical Device Regulation (EU MDR) (2017/745) was approved by the European Parliament on May 25, 2017. This new medical device change recognizes historical medical device problems and aims to strengthen regulatory requirements and oversight to foster a more FDA-like regulatory environment. The mandatory deadline for compliance is fast-approaching...don't miss it!
We are currently offering a Free 30 Minute Consultation to help you navigate EU MDR. To schedule your Free 30 Minute Consultation, just fill out the form, and one of our team members will be in touch to set up your meeting.
Don’t wait to schedule your Free 30 Minute Consultation! This service is only available for a limited time.
Let PRP provide the guidance you need on what to expect. Our subject matter experts can help you navigate these changes by sharing how other medical device companies are approaching these challenges. To schedule your Free 30 Minute Consultation, just fill out the form. One of our Client Service Managers will be in touch to schedule a 10-minute call to discuss how we are helping our clients with these issues as well as to set up your 30 Minute Consultation.
Don’t wait to schedule your Free 30 Minute Consultation!
Since 2012, Professional Resource Partners (PRP) has been honored to serve as a trusted source of quality and regulatory compliance consultants to the medical device and diagnostics industries. In 2017, we launched PRP Quality which specializes in Automotive Quality inspection, sorting, and containment. As an ISO:9001 Certified Business and a WBENC Certified Woman-Owned Business, our company is proud to deliver exceptional professional integrity and service to the many clients we support with our customized approach. It’s also the reason why, when PRP is involved, client expectations are not simply met, but they are consistently surpassed.

